Bone marrow is the body’s most prolific organ. It produces on the order of 400 billion myeloid cells daily, all of which originate from a small number of pluripotent stem cells (Figure 6.3). The bone marrow is comprised of 500 to 1,000 billion cells and regenerates itself every two to three days, which represents normal hematopoietic function. Individuals under hematopoietic stress, such as systemic infection or sickle cell anaemia, will have blood cell production rates that exceed the basal level. The prolific nature of bone marrow cells makes it especially susceptible to damage from radio- and chemotherapies. Bone marrow damage limits the extent of these therapies, and some regimens are fully myoablative. Without any hematopoietic support, patients who receive myoablative dose regimens will die due to hematopoietic failure.
Bone marrow transplantation (BMT) was developed to overcome this problem. In an autologous setting, the bone marrow is harvested from the patient prior to radio- and chemotherapies. It is cryopreserved during the time period that the patient undergoes treatment. After chemotherapeutic drug application and several half-lives of the drug have passed, the bone marrow is rapidly thawed and returned to the patient. The bone marrow cells are simply introduced into the circulation, and the bone marrow stem cells naturally “home” to the marrow cavity and reconstitute bone marrow function. In other words, the hematopoietic tissue is rebuilt in vivo by these cells. This process takes several weeks to complete, during which time the patient is immunocompromised.
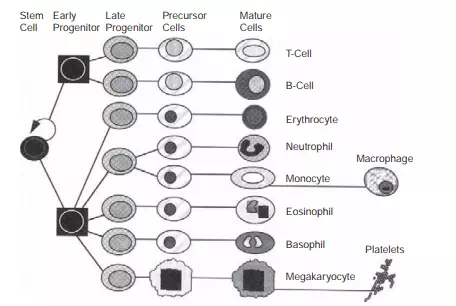
FIGURE 6.3 Hematopoietic cell production. The production fluxes through the lineages can be estimated based on the known steady-state concentration of cells in circulation, the total volume of blood, and the half-lives of the cells. Note that the 400 billion cells produced per day arise from a small number of stem cells.
Current forms of autologous BMT as a cellular therapy simply involve removing the cells from the patient and storing them temporarily outside the patient’s body. There are several advantages to growing and increasing the number of harvested cells, and therefore newer therapies and treatments are being developed based on ex vivo culture of hematopoietic cells. In addition, new techniques to harvest bone marrow stem cells have been developed. These methods rely on using cytokines or cytotoxic agents to “mobilize” the stem cells into circulation. The hematopoietic stem and progenitor cells are then collected from the circulation using leukapheresis.
Myoablative regimens are used in allogeneic settings. In the case of leukaemia, this not only removes the bone marrow but hopefully also the diseased tissue. The donor’s cells migrate to the marrow and repopulate the bone cavity, just as in the autologous setting. The primary difficulty with allogeneic transplants is high mortality (10 to 15 percent), primarily due to Graft-versus-Host Disease, in which immune cells in the transplanted marrow recognize the recipient’s tissues as foreign and mount an immunologic attack. Overcoming this rejection problem would significantly advance the use of allogeneic transplantation in BMT as well as other cell-based therapies.
BMT is a well-developed and accepted cellular therapy for a number of indications. These include allogeneic transplants for diseases such as leukaemia and autologous transplants for diseases such as lymphoma and breast and testicular cancer. Significant growth has occurred in the use of BMT since the mid-1980s, and tens of thousands of patients are now treated using this family of therapies each year. Improvements continue to be made in tissue harvesting, processing, and transplantation, and these advances help to inform the field of tissue engineering as new cell-based therapies are developed.
