What Is Tissue Engineering?
Tissue engineering is a biomedical engineering discipline integrating biology with engineering to create tissues or cellular products outside the body (ex vivo) or to use the gained knowledge to better manage the repair of tissues within the body (in vivo). This discipline requires understanding of diverse biological fields, including cell and molecular biology, physiology and systems integration, stem cell proliferation and differentiation, extracellular matrix chemistry and compounds, and endocrinology. It also requires knowledge of many engineering fields, including biochemical and mechanical engineering, polymer sciences, bioreactor design and application, mass transfer analysis of gas and liquid metabolites, and biomaterials. Translation of tissue engineering constructs to clinical applications will involve yet other scientific disciplines so novel engineered tissues will be easily accepted and used by clinicians. The combination of these sciences has spawned the field of regenerative medicine, which is closely aligned with tissue engineering but has a focus on strategies that use the body’s natural regeneration mechanisms to repair damaged tissues. Two of the main goals of these fields are cell therapies for the repair of damaged tissues, involving injection or engraftment of cells or cellular suspensions, sometimes in combination with scaffolding material, or establishing tissue ex vivo for use as grafts or extracorporeal organs to assist or supplement ailing in vivo organs. Clinical trials with cell therapies or extracorporeally created tissues have begun to be undertaken in the area of skin, cartilage, bone, heart, neural, and liver tissues, and the first tissue-engineered products have become available in the last decade. In addition, tissue engineering strategies are being employed to develop improved in vitro diagnostic and screening techniques, as well as creating improved tissue models to study disease. Both scientific and economic issues will define the success of these and future therapeutic modalities.
The Challenges Facing the Tissue Engineer
Some of the fundamental challenges that face the tissue engineer “in the implementation of cell therapies or creation of grafts and bioartificial organs” are shown in Figure 6.1. In particular, the following issues will impact the field as it progresses toward larger-scale clinical application:
1. Reconstitution of physical (mass transfer) and biological (soluble and insoluble signals) microenvironments for the development and control of tissue function.
2. Overcoming scale-up challenges in order to generate cellular microenvironments on a clinically and commercially meaningful scale.
3. Systems automation to provide appropriate process and quality control on clinically and commercially meaningful scales.
4. Implementation of tissue engineering technologies in clinical settings, including appropriate cell handling and preservation procedures that are required for cell therapies and the transplantation of viable tissues.
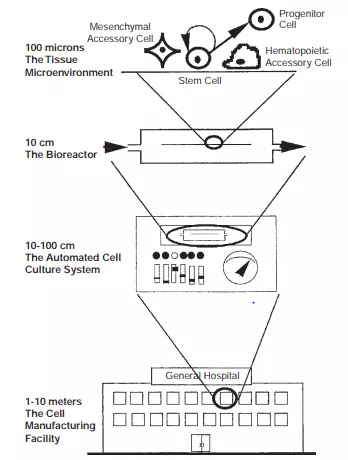
FIGURE 6.1 The four principal size scales in tissue engineering and cellular therapies.
This chapter concentrates on items 1 and 2, although some of the challenges faced with items 3 and 4 are discussed in Section 6.6. For an overall understanding, items 1 and 2 are further illustrated in Figure 6.2 from the viewpoints of “every cell in the body.”
In the centre of Figure 6.2 is a cell. The figure represents the environment that influences every cell in the body. This environment includes the chemical components of the microenvironment: the extracellular matrix, hormones, cyto/chemokines, nutrients, and gases. Physically, it is characterized by its geometry, the dynamics of respiration, and the removal of metabolic by-products. From these characterized observations, expanded details of each component will be discussed such that biological understandings precede the physical considerations. Finally, both biological and physical environments are combined to help integrate research and clinical activities—for the future development of tissue engineered products.
Cellular Therapies, Grafts, and Extracorporeal Bioartificial Organs
The development of cellular therapies initially arose from advancing knowledge within the cell and molecular biology science domains. Transferring these developments into the clinical arena is a design challenge that requires organized culture control and exploitation
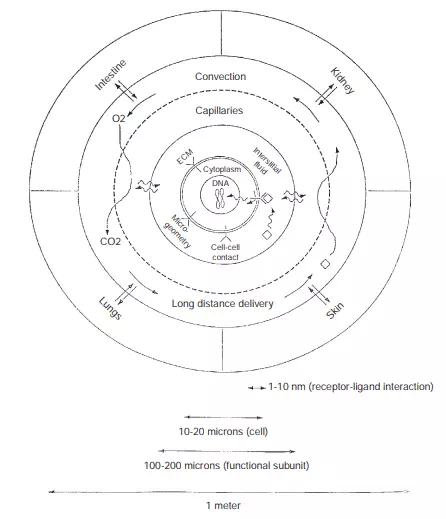
FIGURE 6.2 A cell and its communication with other body parts.
of cell metabolites. For this reason, many scientific fields such as bioengineering, biochemical engineering, and biomaterial sciences are needed for the implementation of cell therapies. A significant challenge in tissue engineering is isolating and growing sufficient numbers of cells for device/therapy designs for clinical and commercial programs. As a historical example, it should be noted that the discovery of penicillin alone was not enough to affect the delivery of health care. Methods for the mass production of clinical grade material had to be developed. The development of such large-scale production of antibiotics arguably represents the most significant contribution of engineering to the delivery of health care. In a similar fashion, the development of industrial-scale methods for isolation, expansion, and cryopreservation of human cells will enable routine uses for cell therapies. In order for tissue engineering to have a tangible impact on modern medicine, the therapies that are developed must be both scientifically and commercially viable.
Tissues are comprised of multiple cell types that interact dynamically with each other. Therefore, tissue-specific functions are often observed only with cocultures of those multiple cell types or with cultures of a particular cell type in combination with the signals from the others. Those signals include insoluble factors in the extracellular matrix, signals from direct cell-cell contact, and soluble signals from autocrine, paracrine, and endocrine interactions. To use these signals as bioengineers, several basic concepts in cell biology need to be understood and quantitatively characterized. These include the key cellular processes of cell differentiation, hyperplastic and hypertrophic growth, migration (motion), protein synthesis, and death (necrosis or apoptosis), all of which combine to define tissue function. Basic information about stem cell and maturational lineage biology and the role of determined stem cells in organ function, genesis, and repair will be presented.
The creation of new engineered tissues requires that many bioengineering challenges be met. For example, bioengineering considerations in cell therapies include injection needle design and procedure protocols. For this application, needles must be optimized to reduce shear stress on cell membranes. Nutrient mass transfer must be analyzed to determine the range of cell aggregate sizes that can be sustained as viable tissues. Engraftment techniques and seed site selection criteria must be established so cells will prosper and assist in system homeostasis. Detrimental events, such as the formation of emboli, need to be prevented. For more complex implantable devices and bioreactors, other challenges will be faced. In these systems, the function, choice, manufacturing, and treatment of biomaterials are important for cell growth and device construction. Fluid mechanics and mass transfer play important roles in normal tissue function and therefore become critical issues in ex vivo cellular device designs. System analysis of metabolism, cell-cell communication, and other cellular processes can be used to define bioartificial organ specifications. A properly designed ex vivo culture system must appropriately balance the rates of biological and physicochemical processes to obtain desired tissue functions. By mathematically modeling this balancing effect, dimensionless parameter groups can be formulated that describe characteristic ratios of time constants. In this way, new dimensionless values will evolve to relate ratios of “physical times” with “biological times.”
Finally, the implementation of cell therapies and tissue grafts in the clinic requires the recognition and resolution of several difficult issues. These include tissue harvest, cell processing and isolation, safety testing, cell activation/differentiation, assay and medium development, storage and stability, and quality assurance and quality control issues. These challenges will be described in this chapter but are not analyzed in detail.
Human Cells and Grafts as Therapeutic Agents
Cell therapies use human cells as therapeutic agents to alleviate a pathological condition. It is important to note that some cell therapies are already an established part of medical care. One existing type of cell therapy is blood transfusion, which has been practiced for decades with great therapeutic benefit. This therapy uses red blood cells (RBC) as the transplant product into anaemic patients to help to restore adequate oxygen transport. Similarly, platelets have been transfused successfully into patients who have blood clotting problems. Bone marrow transplantation (BMT) has been practiced for almost two decades, with tens of thousands of cancer patients undergoing high-dose chemo- and radiotherapies followed by BMT. More recently, transplantation of hemopoietic stem cells has occurred with increasing frequency to correct haematological disorders. These are all applications of cell therapies associated with blood cells and blood cell generation (haematopoiesis). (The term haematopoiesis comes from the Greek hemato, meaning “blood,” and poiesis, meaning “generation of.”) Therefore, a large population of patients already has benefitted from cell therapies, and this benefit can be extended by developing new therapies using other progenitor cell sources.
Transplants can be xenogeneic (donor and recipient are members of different species), allogeneic (donor and recipient are members of the same species but are not genetically identical), or syngeneic (donor and recipient are genetically identical—e.g., clones in the case of animals, or identical twins). Syngeneic transplants include autologous transplants (cells from a patient being isolated and given back to the same person). The issues associated with allogeneic transplants are well known because of the widespread use of organ transplantation and chiefly involve prevention of immune rejection as well as longer-term negative responses to transplanted tissues. However, with the advent of ex vivo cell culture and advances in cell manipulation procedures, autologous transplantation is becoming more common. In addition, there are efforts under way in several laboratories to create “universal donor” cell sources and cell lines that would alleviate many of these issues.
The ability to reconstitute tissues ex vivo and produce cells in clinically meaningful numbers has broad implications. Table 6.1 summarizes the supply and demand of organs and tissues versus the number of procedures performed annually in the United States. Although the number of procedures is limited, the overall cost of these procedures was still estimated at a staggering $400 billion per year. The potential socioeconomic impact
TABLE 6.1 Incidence of Organ and Tissue Deficiencies, or the Number of Surgical Procedures Related to These Deficiencies in the United States

TABLE 6.1 Incidence of Organ and Tissue Deficiencies, or the Number of Surgical Procedures Related to These Deficiencies in the United States

of cellular therapies is therefore substantial. Progress in decreasing the costs of these therapies also will encourage investment into new types of treatments. In this way, new medical products will be developed to greatly improve the quality of life and productivity of affected individuals.
The concept of directly engineering tissues was pioneered by Y. C. Fung in 1985. The first symposium on this topic was organized by Richard Skalak and Fred Fox in 1988, and since then the field of tissue engineering has grown rapidly. Thousands of scholarly articles have been written on the topic, and in 1995 a peer-reviewed journal called Tissue Engineering was established. Since that time, the original journal has sprouted into three separate journals (Part A: Primary Papers, Part B: Reviews, and Part C: Methods) to more broadly cover the field, and numerous other journals in the topic area have emerged, including the Journal of Tissue Engineering and Regenerative Medicine. The field also has received considerable attention in the lay press because of the opportunities it presents to revolutionize medicine for an aging population.
The last two decades have seen remarkable advances in biology that have enabled tangible progress in tissue engineering. Cutting-edge cell therapies that have reached the advanced stages of development include various forms of immunotherapies, chondrocytes for cartilage repair, liver and kidney cells for extracorporeal support devices, b-islet cells for diabetes, skin cells for patients with ulcers or burns, and genetically modified myocytes for treatment of muscular dystrophy. In addition, engineered tissues such as blood vessels, bladders, urethras, and other tissues are rapidly moving toward the clinic. As would be expected based on tissue complexity, the challenges faced with each tissue are different. A few examples are provided in the following sections for illustrative purposes.
