. 
Tissue Engineering is an interdisciplinary discipline addressed to create functional three-dimensional (3D) tissues combining scaffolds, cells and/or bioactive molecules. Tissue Engineering is the application of science to improve, restore and maintain the damaged tissues or the whole organ. It makes tissues functional by combining scaffolds, cells and biologically active molecules. Although it was considered to be a subfield of biomaterials, it has emerged widely on its own.

Tissue engineering is a specialized branch under biomedical engineering (bioengineering). This field involves scientific areas such as cell biology, material science, chemistry, molecular biology, and medicine. Tissue engineering evolved from the field of biomaterials development and refers to the practice of combining scaffolds, cells, and biologically active molecules into functional tissues.

Tissue engineering is continuously evolving assimilating inputs from adjacent scientific areas and their technological advances, including nanotechnology developments. The objective of tissue engineering is to assemble functional constructs that restore, maintain, or improve damaged tissues or whole organs.
Tissue Engineering Market
The global tissue engineering market size was valued at around USD 5 billion in 2016 and is expected to expected to reach USD 11.5 billion by 2022, according to a new report by Grand View Research, Inc. Growing potential of tissue engineering procedures in the treatment of tissue damages is supporting the market growth. Tissue engineering can provide solutions that can replace the currently used tissue repair solutions including transplants, surgical reconstruction, and mechanical devices.
The most key application segments of tissue engineering are Cancer, cord blood & cell banking, GI & gynecology, skin or integumentary, dental, urology, musculoskeletal, orthopedics, spine, cardiology & vascular and neurology.
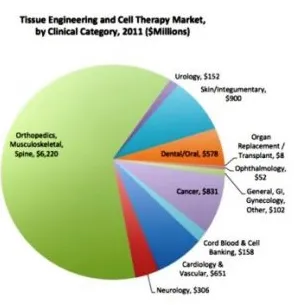
 Tissue Engineering Market in the US
Tissue Engineering Market in the US
North America accounted for the largest share in 2015 and is likely to continue its dominance till 2023, driven by the U.S. tissue engineering market. The growth can be attributed to the high financial support from the government as well as private organizations for research and development and presence of leading companies.
Europe tissue engineering market is estimated to record moderate growth prospects over the forecast period due to the presence of strict regulatory norms. Constant research & development in regeneration surgeries, as well as stem cell research, is projected to offer massive growth opportunities, over the next seven years.
The Asia Pacific is forecast to be the most attractive region over the next seven years. The regional growth has been fuelled by massive manpower, technologically advanced procedures, and government support & funding.
Major global players in the tissue engineering industry include Zimmer Biomet, Medtronic, Acelity, Organogenesis, Athersys, Stryker and RTI surgical. Other prominent companies are Integra LifeSciences, Advanced Cell Technology, CryoLife, Sanofi, BioMimetic Therapeutics, StemCellsInc, LifeCell Kinetic Concepts, Cook Biotech, and Arteriocyte.
So, the market demand and scopes of tissue engineering are pretty clear. Now, we will dive into the basic concepts of tissue engineering. We will start with biomaterials since it’s a basic component of tissue engineering.
Biomaterials
Biomaterials form an integral component in Tissue Engineering. Many of the materials have been found to be of use in tissue engineering. Biomaterials are either used for therapeutic or diagnostic purposes. Biomaterials and biological materials are two different concepts. A biomaterial is said to be an ideal one which fulfills the following requirements:
· injectability
· synthetic manufacture
· biocompatibility
· non-immunogenicity
· transparency
· nano-scale fibers
· low concentration
· resorption rates
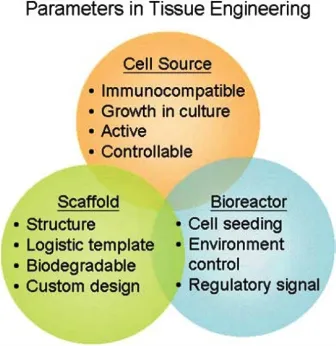
1. Scaffolds– Scaffolds are materials engineered for the formation of new functional tissues and used for medical purposes. Scaffolds recreate the in-vivo environment that is provided by the extracellular matrix. Depending on its origin, Scaffolds are classified into two types. Natural scaffolds take part in the process of morphogenesis and function acquisition of different cell types in the in-vivo environment. The composition of these scaffolds depends on animal origin, isolation and purification procedures, and assays. Synthetic scaffolds are made to mimic specific ECM(Extra Cellular Matrix) properties under controlled conditions.
Uses of scaffolds include-
· cell attachment and migration
· retention of cells and biochemical factors
· allowance for the diffusion of vital cell products and expressed products
· modification of the behavior of cell phase by exerting biological and mechanical influences.
Scaffolds need to fulfill specific requirements for tissue engineering. They are:
· An adequate pore size with high porosity for facilitating cell seeding and diffusion into the whole structure.
· Biodegradability is one of the factors. The scaffold should provide structural integrity while cells are fabricating their natural matrix structure around themselves. It should break down as soon as the new tissue forms. The degradation has to coincide with the rate of tissue formation.
The three types of biomaterials that are used for fabrication of scaffolds are:
Ceramics– They have excellent biocompatibility because of their chemical and structural similarity. They constitute high mechanical stiffness and very low elasticity. Examples include- hydroxyapatite (HA) and tri-calcium phosphate (TCP), for bone regeneration applications.
Synthetic polymers– They exhibit controlled degradation characteristics and are easy to be fabricated with a tailored architecture. Examples include- polystyrene, poly-l-lactic acid (PLLA), polyglycolic acid (PGA) and poly-dl-lactic-co-glycolic acid (PLGA).
Natural polymers– They are biologically active and allow host cells to produce their own extracellular matrix and replace the degraded scaffold.
2. Cells– Cell source selection is important for tissue engineering. But, a difficulty is encountered which lies in growing specific types of cell in large quantities. Therefore, stem cells ( Embryonic or Adult Stem Cells) have emerged as promising alternative cell sources. ESC’s are pluripotent cells whereas ASC’s are multipotent cells. ASC’s are more appropriate for Tissue Engineering as they have a more limited capacity to differentiate than ESCs.
3. Biomolecules– Signalling molecules are much as important as the scaffolds and cell source. These signals are unique to each organ and are tightly controlled. The presence of factors such as growth factors, chemokines, and cytokines play an important role in biological phenomena. The use of the signaling molecules can be in two ways that are- addition to the culture media in-vitro or attachment to the scaffold by covalent and non-covalent interactions.
Application of Tissue Engineering
How Tissue Engineering and Regenerative Medicines work?
Regenerative Medicines repair damaged tissues and organs. They stimulate the body’s own repair mechanisms to heal previously irreparable tissues or organs. If the body cannot heal itself, the tissues or organs can be grown in the laboratory and then implanted. Regenerative Medicine also involves the use of stem cells or progenitor cells obtained through directed differentiation.
The process begins with the creation of scaffolds and introducing cells into it. A tissue develops once it gets the right environment. In some cases, self-assembly occurs which involves the mixing of all the cells, scaffolds, and growth factors together.
Another approach can be by stripping the cells of a donor organ and using the remaining collagen scaffold to grow new tissue. This approach has been a promising one to bioengineer heart, liver, lung, and kidney tissue.
Some key research areas include-
Implantation of human liver in mice: The implantation of the engineered human liver into the mice can make the drug interactions similar to that happens in the human system. The test of toxicity, species-specific responses can be easily understood by the researchers.
Regeneration of a new kidney: The kidney scaffolds seeded with epithelial and endothelial cells developed in organ tissue. The tissue produced urine both in-vitro and in-vivo in rats. The ability to regenerate a new kidney is a leap forward in overcoming the problems of donor organ shortages.
How does Tissue Engineering help in Regeneration of Damaged Tissues?
The applications of Tissue Engineering have been helpful in overcoming problems of any damaged tissues.
Bone Tissue Engineering- Bones are composed of collagen and have the property to regenerate, repair in response to an injury. The requirement of bone graft takes place during large bone defects occurring after trauma, infection, tumor resection or skeletal abnormalities.
Producing the features of bones in-vitro is very challenging. So to obtain an ideal scaffold for bone tissue regeneration is also difficult. Scientists have been able to develop 3D porous scaffolds with similar composition to the bone and, for better compatibility, Bioceramic scaffolds are used. The osteo-inducive scaffolds make use of biomolecular signaling and progenitor cells for new bone formation. In the bone defect models, the nanoparticles designed for the release of osteogenic factors showed increased in-vitro and in-vivo osteogenic differentiation.

Cartilage tissue engineering- Cartilage is a connective tissue found in elbows, knees, and ankles. Like bone tissue engineering, challenges also lies with cartilage tissue engineering. Several scaffolds have been used for cartilage repair but, the most relevant are the synthetic scaffolds like polyurethane, Poly (Ethylene Glycol) (PEG), elastin-based polymers. Cartilage is composed of chondrocytes so, an ideal donor cell type for cartilage repair is autologous chondrocytes. However, they are difficult to obtain and require invasive techniques. Therefore, Mesenchymal Precursor Cells (MSCs) collected from different sources, such as adipose tissue or bone marrow have been used as an alternative source. They can be easily cultured in-vitro and have the ability to proliferate and differentiate towards osteogenic, adipogenic, chondrogenic and myogenic lineages.
Apart from bone and cartilage tissue engineering, certain other TE like cardiac tissue engineering, pancreas tissue engineering, vascular tissue engineering has also been done.
In-Vitro Human Models for Tissue Engineering
Creation of In-Vitro Human Models for Tissue Engineering is another application to analyze the role of different chemical, mechanical and/or physical factors in a simple system.
Cancer- To recreate the process of tumor progression, an accurate modeling of tumor microenvironment is required. This is possible through 3D cultures that can provide the micro-environmental conditions that control tumorigenesis. The 3D cultures are based on combining cells, scaffolds, and biomolecules. Both natural and synthetic biomaterials have been used to model cancer.
Drug Discovery- For effective drug screening, 3D cultures are introduced to analyze the effect of drug action. Hepatocytes regained their morphology and expression of key-liver proteins when cultured in 3D culture.
Even if Tissue Engineering is life-saving, it is expensive. Allografts and transplants face risks of getting rejected by the patient’s system. Further research is still to be carried out. However, advances in areas of bone, cartilage, heart, pancreas, and vasculature have taken Tissue Engineering to a new level. Tissue Engineering is transforming biology and technology by making a deep impact on the development of new therapies.
Future Scopes of Tissue Engineering
At present, tissue engineering plays a relatively small role in patient treatment. Artificial skin, valves, and cartilage are examples of engineered tissues that have been approved by the FDA. However, currently, they have limited use in the humans.
Supplemental bladders, small arteries, skin grafts, cartilage, and even a full trachea have been implanted in patients, but the procedures are still experimental and very costly. While more complex organ tissues like heart, lung, and liver tissue have been successfully recreated in the lab, they are a long way from being fully reproducible and ready to implant into a patient.
These tissues, however, can be quite useful in research, especially in drug development. Using functioning human tissue to help screen medication candidates could speed up development and provide key tools for facilitating personalized medicine while saving money and reducing the number of animals used for research.

Comments are closed.